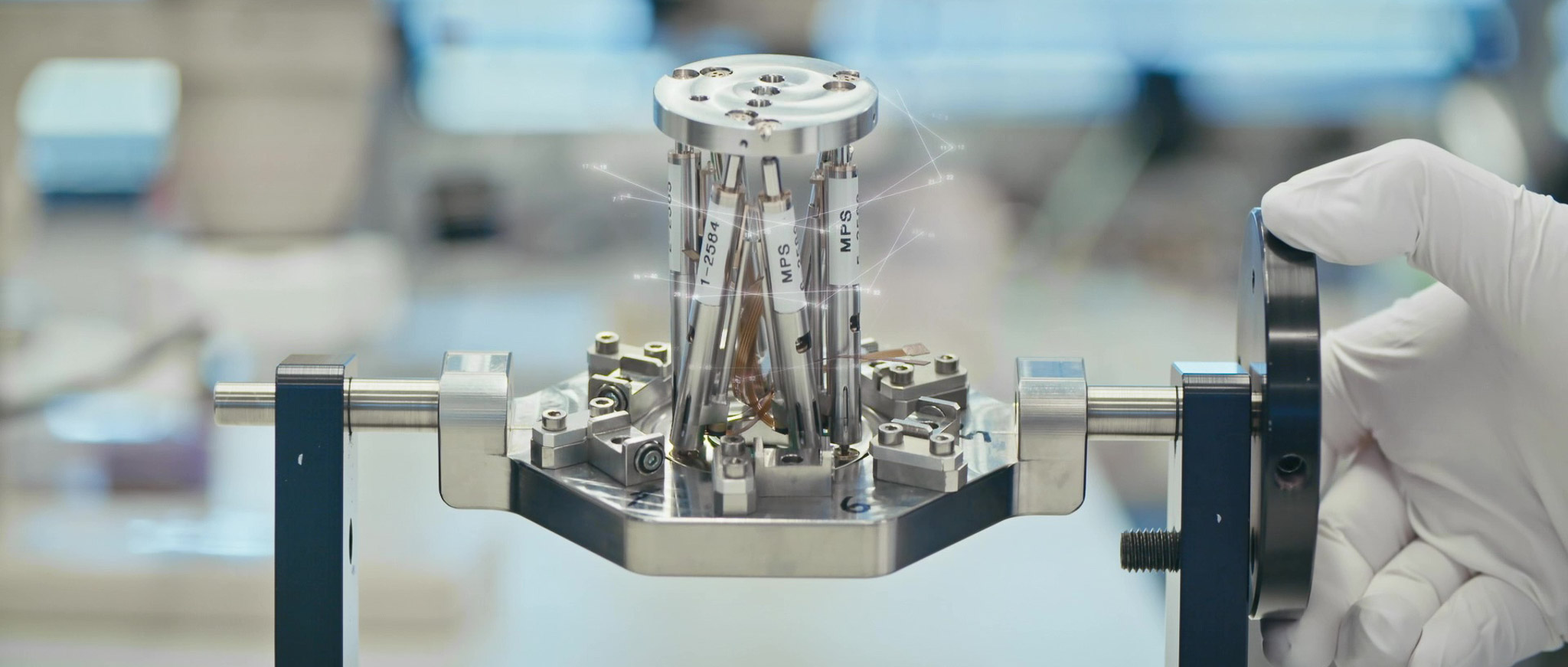
Competences
With a large number of multidisciplinary teams and a network of trusted partners, MPS manages product development projects from idea to series production. We are the single point of contact for our customers, which makes project management much easier and more efficient.
Our various departments of R&D, process engineering, production, assembly, quality engineering and validation work hand in hand with project managers to develop and produce solutions that meet the demanding needs of our customers.


Project management
MPS manages your project from idea to mass production. We are driven by customer satisfaction and business success, focusing on quality, communication, cost and time. Taking responsibility for the entire project, we differentiate ourselves from our competitors by being the single point of contact for customers. MPS manages international multidisciplinary project teams, working with R&D, industrialization, quality and purchasing engineers to deliver innovative and efficient solutions.
Innovation & development
At MPS, innovation and the pursuit of excellence are at the heart of our philosophy. By constantly pushing the limits of what is possible, we develop innovative solutions to meet the specific needs of our customers. With its R&D team of specialized engineers, MPS designs high-performance ball bearings, electromechanical systems and microtechnical devices. In addition, we have a competent and flexible process engineering department to transform our ideas into solid and repeatable manufacturing and assembly processes.
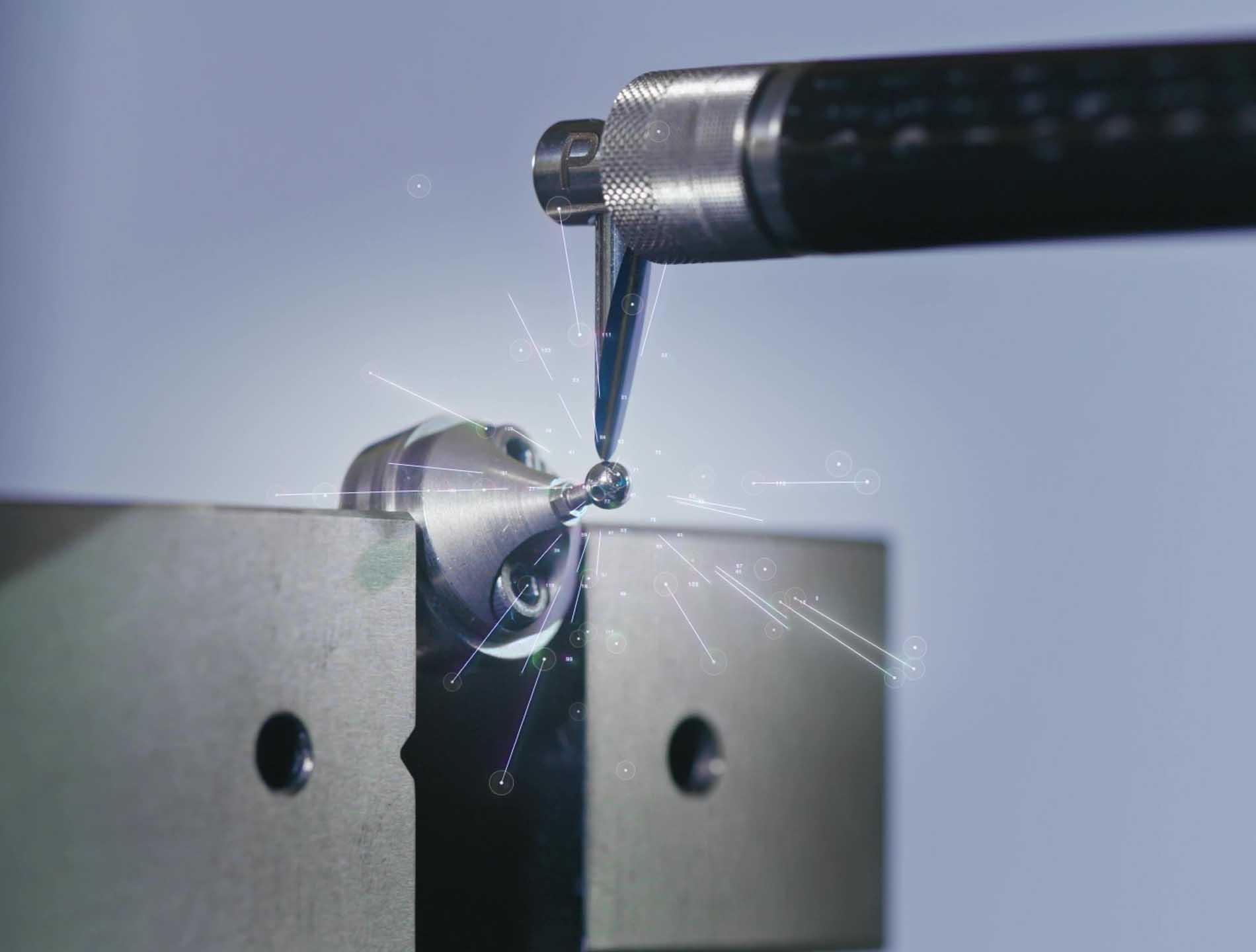

Production & assembly
Our three production sites in Biel, Bonfol and Court are equipped with the latest technology. These state-of-the-art facilities enable us to produce a wide range of complex and small parts. Thanks to a modern infrastructure, we apply a wide range of machining and assembly processes, including turning, milling, lapping, EDM, welding, polishing and many others. In addition, we assemble high-performance microtechnical systems dedicated to the watchmaking, optical, medical and aerospace markets in our facilities.

Validation
MPS has established validation processes to ensure that all products are designed, manufactured and tested in accordance with regulatory requirements and quality specifications. Validation of a medical product includes several steps such as performance testing, clinical trials, risk analysis and regulatory compliance audits. Our validation team is also responsible for maintaining the validated state of all the company's information systems.