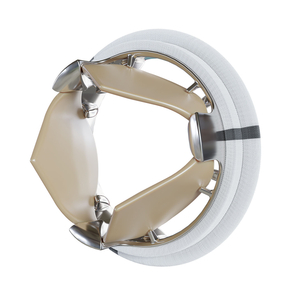
Artificial aortic valve
Novostia, a Swiss startup founded in 2017, has entrusted MPS with the manufacture of its TRIFLO artificial valve, a revolutionary aortic prosthesis on several levels. Thanks to its aerodynamic design, the presence of three leaflets and the absence of physical pivot axes for the leaflets, the TRIFLO valve aims to satisfy the following three criteria:
- A device lifespan at least equal to that of the patient, to avoid the need for a second surgical procedure. This means more than 30 million flap openings and closures per year, with no leaks, blockages, cracks, ruptures or flap escapes.
- The artificial valve must not lead to thrombosis, so that patients do not need to take anticoagulants.
- Operation (flap opening and closing, blood flow through the valve) must be as silent as possible, so as not to disturb the patient or those around him, day or night.
The TRIFLO device is in clinical trial and is not approved for sale.
Characteristics*
- Lifespan at least equal to that of patients
- Superior hemodynamics to current mechanical valves
- Total biocompatibility with blood thanks to the use of implantable medical-grade materials (titanium, PEEK and polyester)
- Low thrombogenicity
- Remarkably silent
- Easily implantable
- Designed for patients of all ages
*only demonstrated in vitro

Exceptional manufacturing processes
The TRIFLO valve offers many advantages over mechanical and biological artificial valves on the market. From the outset of Novostia's development, MPS has been guided by the fundamental requirements determined by the functioning of a human heart.
In addition, to meet stringent tolerances on size, shape, surface finish and appearance, MPS has developed specific manufacturing processes, including milling of three-dimensional shapes to tolerances of less than 10 μm. Watchmaking-quality polishing has resulted in a degree of finish that promotes blood flow without the risk of clot formation.
The successful first clinical trials took place at the end of 2023 (read more), in Vilnius, Lithuania, a city and country that guarantee compliance with the European directive applying to medical devices and the highest quality of care. Marketing of the valve is scheduled for 2028.