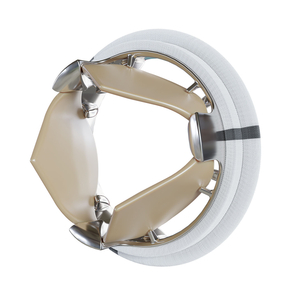
Successful first implantation of the TRIFLO valve
23.01.2024, Lausanne
Novostia, a medical technology pioneer, recently announced the successful first implantation of its TRIFLO valve in a human patient. The operation, which took place in December 2023 at Vilnius University Hospital in Lithuania, is part of a preliminary study to gather data on the safety, performance and efficacy of the TRIFLO valve.
MPS has been entrusted by Novostia with the manufacture of the rigid parts of this valve. The device is intended to be revolutionary, as it aims to be the first to meet the three key criteria that an artificial aortic valve must satisfy:
- The TRIFLO valve is expected to outlast the patient, avoiding the need for a second operation.
- Patients implanted with the TRIFLO valve should not require anticoagulant treatment, as the device has antithrombotic properties by design.
- The TRIFLO valve should to be very quiet, so it won't disturb the patient when the flaps are opened and closed.
Although this is only the first test in a human, Novostia is confident that this device will usher in a revolution in heart valve replacement, given the great benefits that the TRIFLO valve could offer patients.