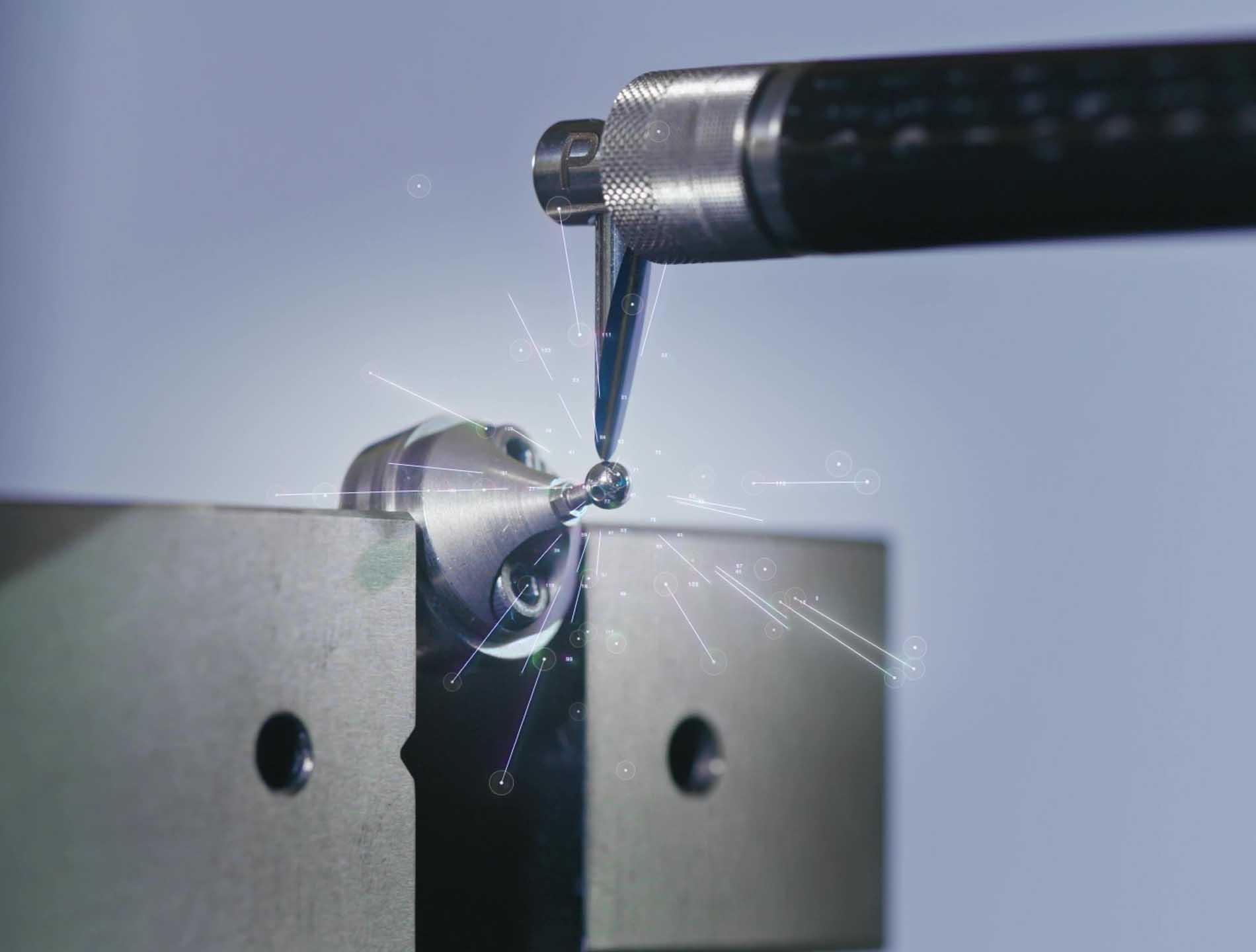
Medical Development and Manufacturing
MPS Microsystems is a CDMO (Contract Development and Manufacturing Organization) that develops and manufactures Class III medical devices for legal manufacturers, delivering highly complex, miniaturized solutions that meet the most stringent technical and regulatory challenges.
We specialize in cutting-edge technologies and collaborate with global partners across multiple continents to create devices that push the boundaries of innovation. Our expertise spans cardiac applications (artificial hearts, pumps, valves), urology (artificial sphincters), orthopedics (bone lengtheners), and spinal implants.
As a full-service partner, we manage the entire project lifecycle from development to production, coordinating our vast network of trusted partners, optimizing supply chains, and integrating parts into subassemblies or ready-to-implant devices.
With ISO 13485 certification, state-of-the-art production, dust-free assembly as well as ISO 5 and 7 cleanrooms, we ensure the highest quality and reliability for life-saving medical innovations.

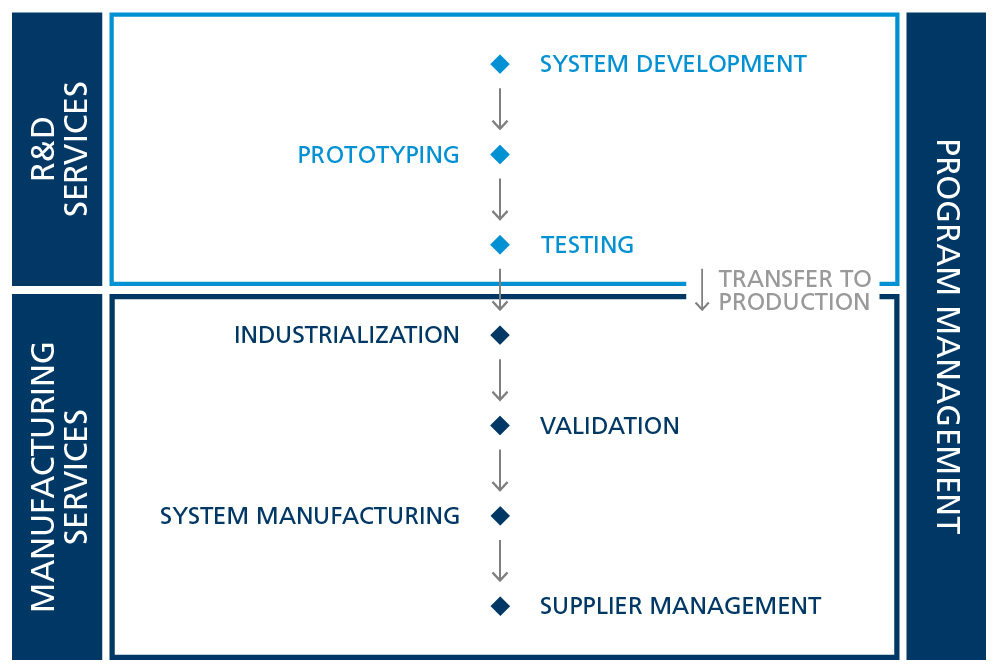
Program management for turnkey medical solutions
When we say we handle everything from A to Z, we mean it.
Our customers value the fact that we are their single point of communication and drive the entire program. In order to do that, we rely on and manage an extensive network of trusted partners with whom we have developed some of our most advanced medical devices and active implants.
Our experienced program managers lead large international medical programs with expertise. We ensure regular, transparent communication with all stakeholders, fostering collaboration and trust. Utilizing an agile matrix organization, we empower decision-making and adapt swiftly to challenges in multidisciplinary, technically demanding projects.

Process innovation
At MPS, we continuously push the boundaries of manufacturing processes while ensuring stability and repeatability. We master advanced machining techniques such as turning, milling, EDM, grinding, polishing, and gluing to produce highly complex components that meet the strict requirements of medical applications.
We also innovate in surface treatments, reducing friction and eliminating debris to enhance the longevity of implantable devices. New materials and fabrication techniques are continuously explored, ensuring optimal performance and reliability.
To guarantee seamless integration and compliance with strict medical regulations, we design custom assembly and testing tools that refine every step of the production process. This commitment to precision, safety, and continuous improvement enables us to deliver cutting-edge solutions that set new industry standards.
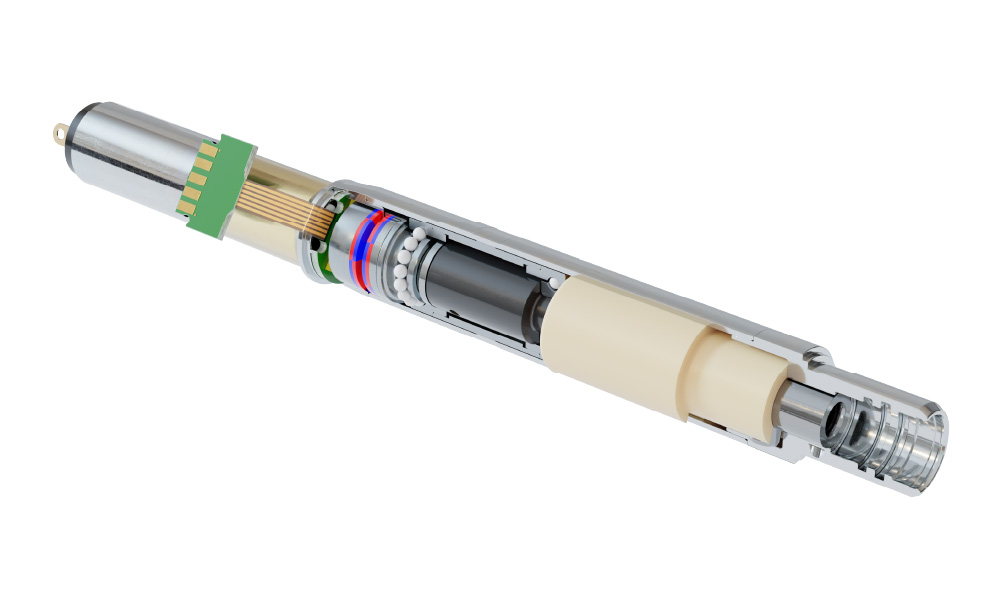
Product innovation
MPS drives product innovation by mastering miniaturized motorized actuation (MMA), enabling precise motion control in ultra-compact medical implants. Our expertise in high-precision micromechanics allows us to develop customized, reliable, and energy-efficient systems, essential for applications where space and performance are critical.
We also specialize in biocompatible materials and advanced surface treatments, ensuring our solutions meet strict regulatory standards for long-term implantability. By leveraging our deep knowledge of material properties, coatings, and sterilization requirements, we create safe, durable, and high-performance medical devices tailored to our clients' needs.
Products
(copy 4)
Implantable peristaltic pump being part of a programmable infusion system capable of storing and delivering pain medication in the intrathecal space
Implantable pump

Operational excellence
Operational excellence is at the heart of MPS, ensuring that every stage of a medical development project, from concept to mass production, meets the highest standards of quality and efficiency. Our commitment to continuous improvement drives us to adopt methodologies like Six Sigma, Lean, and 5S, enabling us to maintain zero-defect production and align with customer growth.
We collaborate closely with clients on value engineering, while also executing internal projects to streamline our processes. A key part of our success is fostering a culture of operational excellence through targeted training, ensuring that every team member is aligned with our objectives. This focus on excellence not only improves efficiency but also ensures a safe, healthy, and fulfilling work environment for our employees.
Quality at heart
Quality lies at the core of everything we do. MPS Microsystems is certified ISO 9001, ISO 13485 and ISO 14001. We conduct an annual review of our certification strategy to maintain compliance with the highest industry standards. Our commitment to quality extends beyond systems and certifications, as we foster a culture of excellence through systematic training, ensuring that every team member contributes to our rigorous quality standards.
Supplier onboarding and integration are meticulously managed to guarantee supply chain security and consistency. Additionally, we prioritize the digitalization of our Quality Management System (QMS) processes, streamlining operations to maintain efficiency and precision across all phases of project development.