Quality
MPS has implemented a quality management system across its entire organisation, which complies with different quality, safety and environmental protection standards. The MPS quality department monitors the company's certifications:
- Certification according to ISO 9001: 2015: quality management system
- Certification according to ISO 13485: 2016: medical devices - quality management system
- Certification according to ISO 14001: 2015: environmental management system
Each of MPS's entities has its own management systems developed in accordance with the guidelines of the departments that govern the group's quality systems. Synergies and joint projects between the various entities of the group enable them to take full advantage of their respective contributions in terms of metrology, quality assurance, control and validation. For each project, all means of production are validated, suppliers qualified and products approved by MPS, according to the customer's specifications. MPS guarantees a high level of quality through the planning of strict and regular controls, which are carried out, for example, in a controlled atmosphere (temperature and humidity). All production monitoring is performed by an SPC (Statistical Process Control) system. MPS is able to develop metrology equipment according to the specific needs of a project.
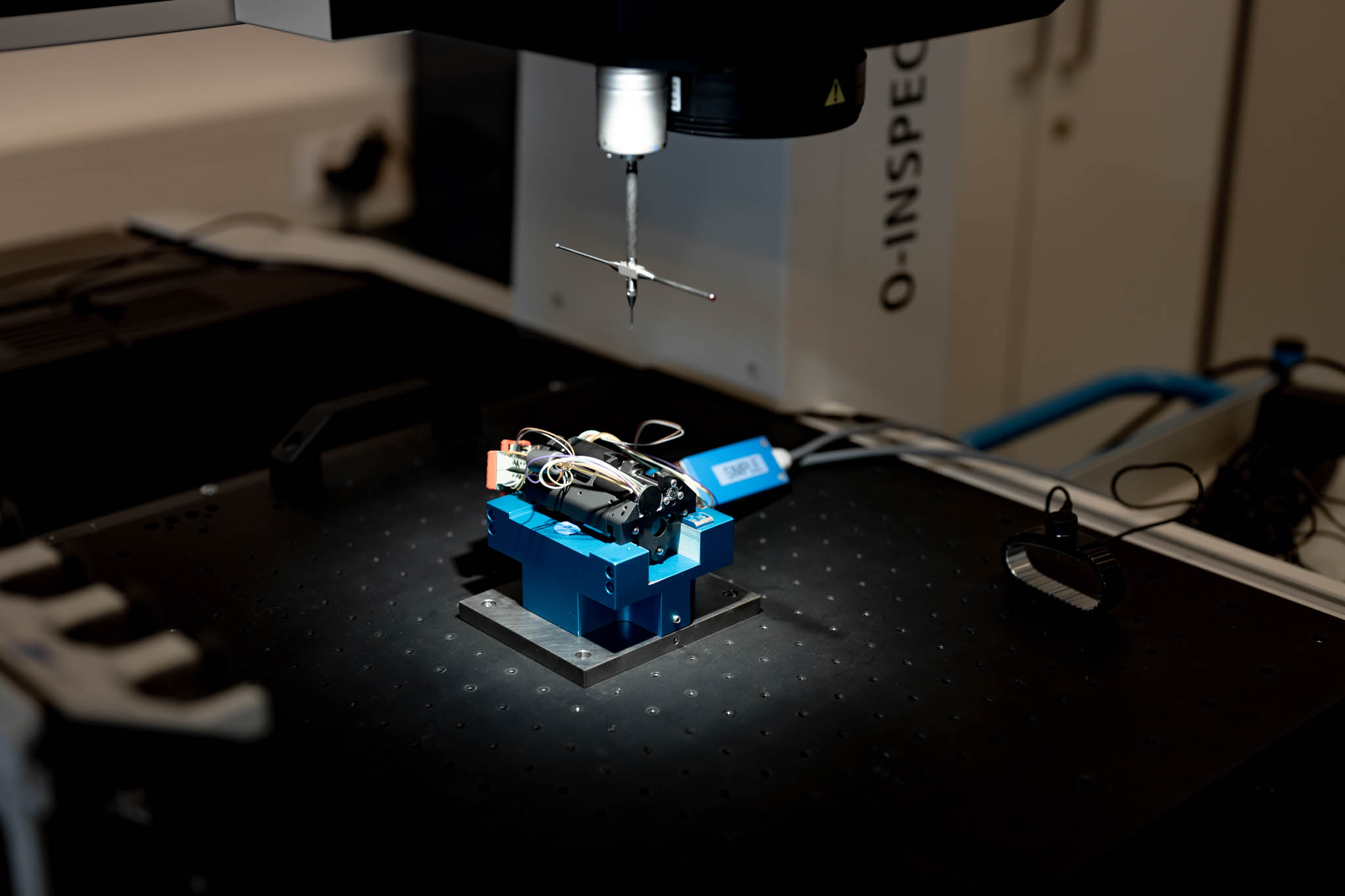
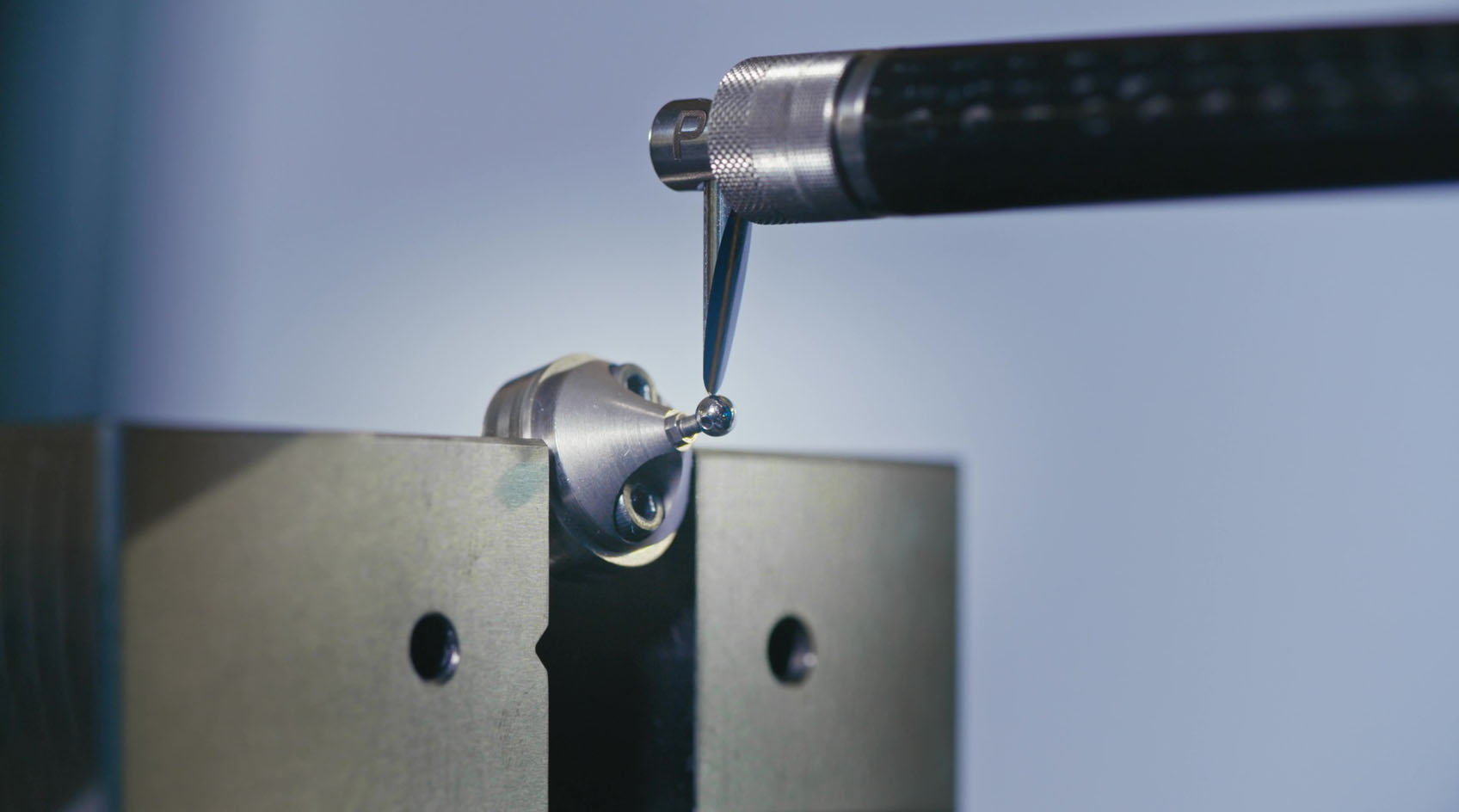
Contrôle et validation
Chacune des entités de MPS dispose de ses propres systèmes de gestion développés selon les directives des services qui régissent les systèmes qualité du groupe. Pour chaque projet, et selon les spécifications du client, tous les moyens de production sont validés, les fournisseurs sont qualifiés et les produits sont homologués par MPS. De ce fait, il est possible de garantir un niveau de qualité élevé grâce à la planification de contrôles stricts et réguliers, qui sont par exemple effectués en atmosphère surveillée (température et humidité). Tout le suivi de production est assuré par un système SPC (Statistical Process Control).
Special needs of the medical field
In the medical field, we guarantee the quality of our products by complying with legal and regulatory requirements through the continuous improvement of our quality systems. Our quality assurance experts ensure that we provide Class I and IIa medical devices for the CE zone or Class I for the FDA zone to our customers.
We prepare the necessary documents for certification and marketing of these medical devices. Our quality management system is certified by the Swiss Association for Quality and Management Systems (SQS) and meets all the requirements of ISO 13485:2012.
- ISO 13485 certificate registration number: 40115
- EU certificate registration number: 41653
- US FDA registration number: 3010045785


Needs for turning processes
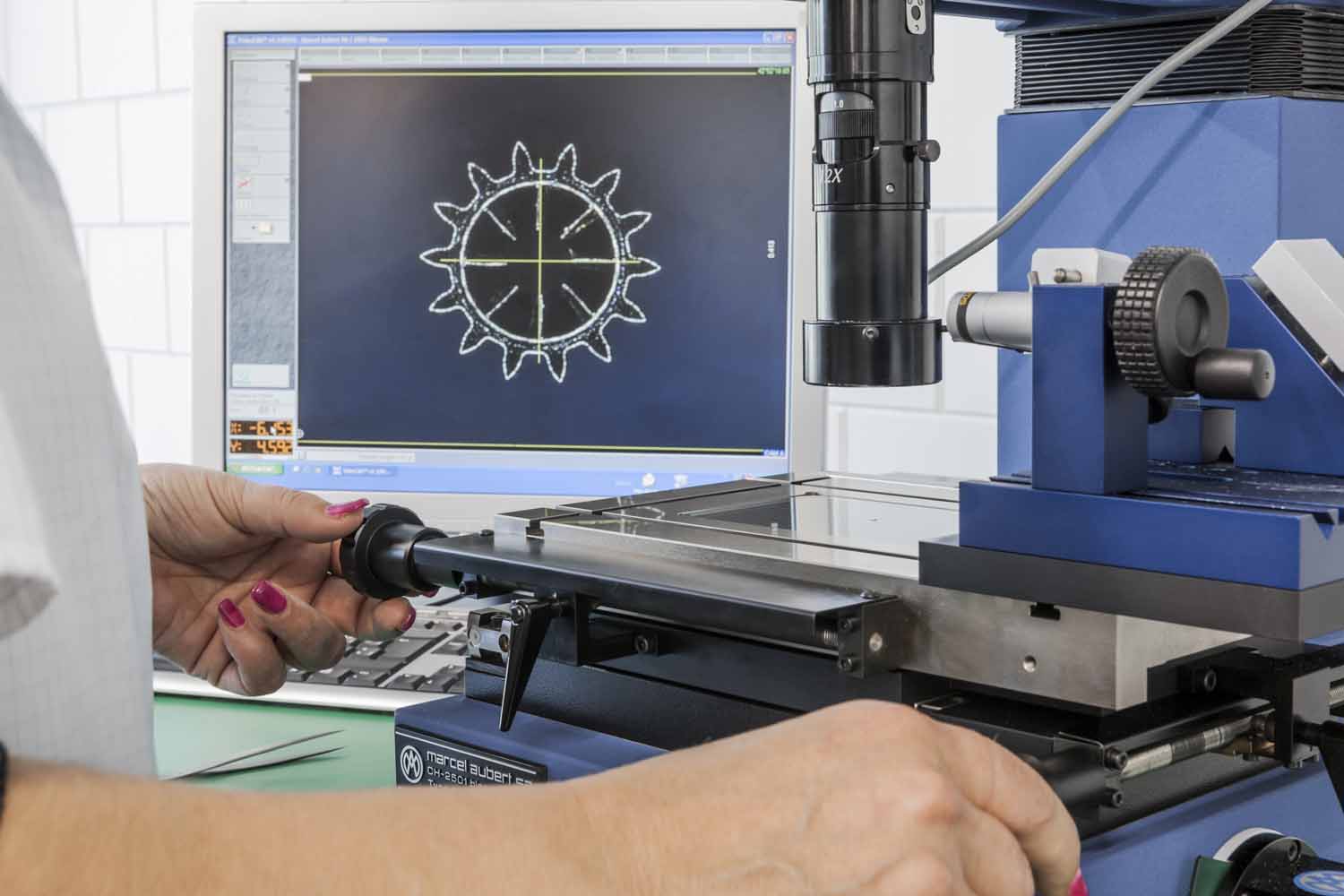
Statistical process control: MPS Décolletage is one of the first turning companies to have introduced the SPC method (Statistical Process Control). Periodic inspections are performed throughout the manufacturing process to ensure full product traceability. Thus, MPS Décolletage can provide the customer, with each delivery, a detailed manufacturing inspection protocol, as well as the different material, treatment and coating certificates.
A clear competitive advantage enabling the company to to eliminate incoming goods inspections and to ensure supply chain security for its customers.